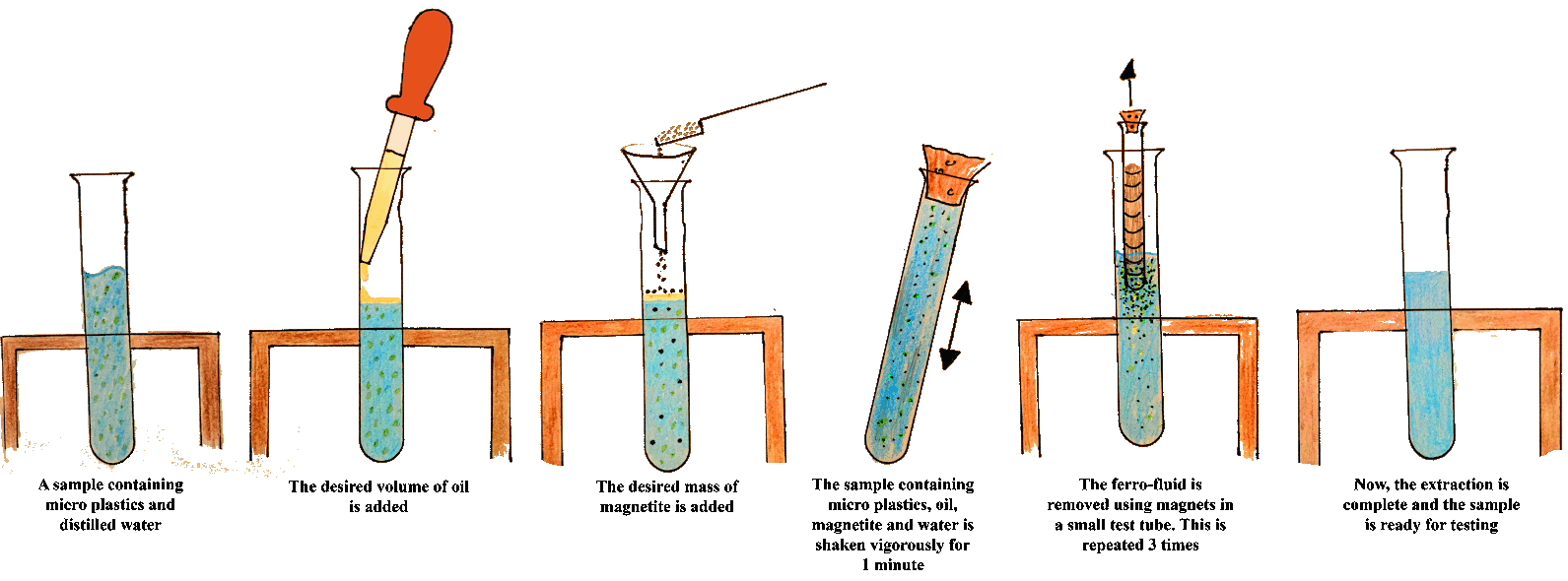|
FIONN FERREIRA MICROPLASTICS CLEANING SYSTEM
PLEASE USE OUR A-Z INDEX TO NAVIGATE THIS SITE OR RETURN HOME
|
|||
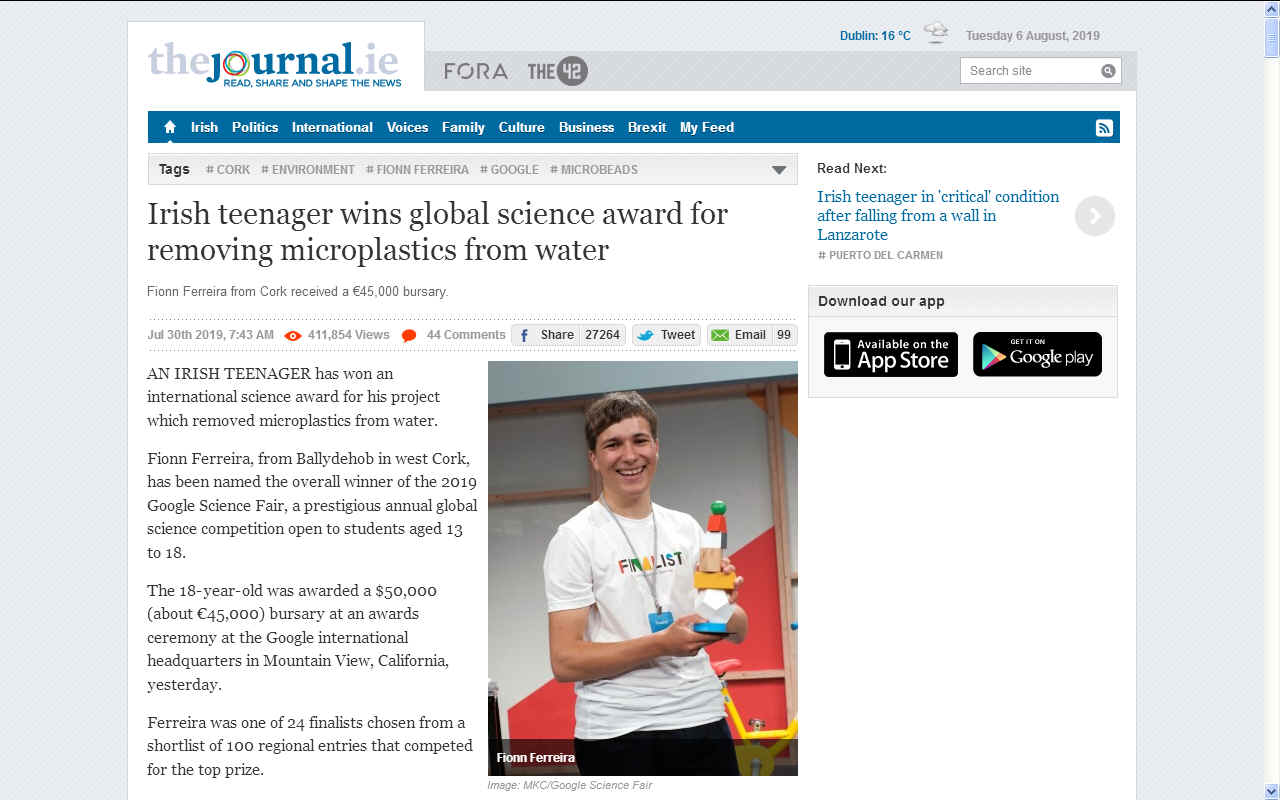
AN IRISH TEENAGER
- has won an international science award for his project which removed microplastics from water.
CNN AUGUST 2 2019
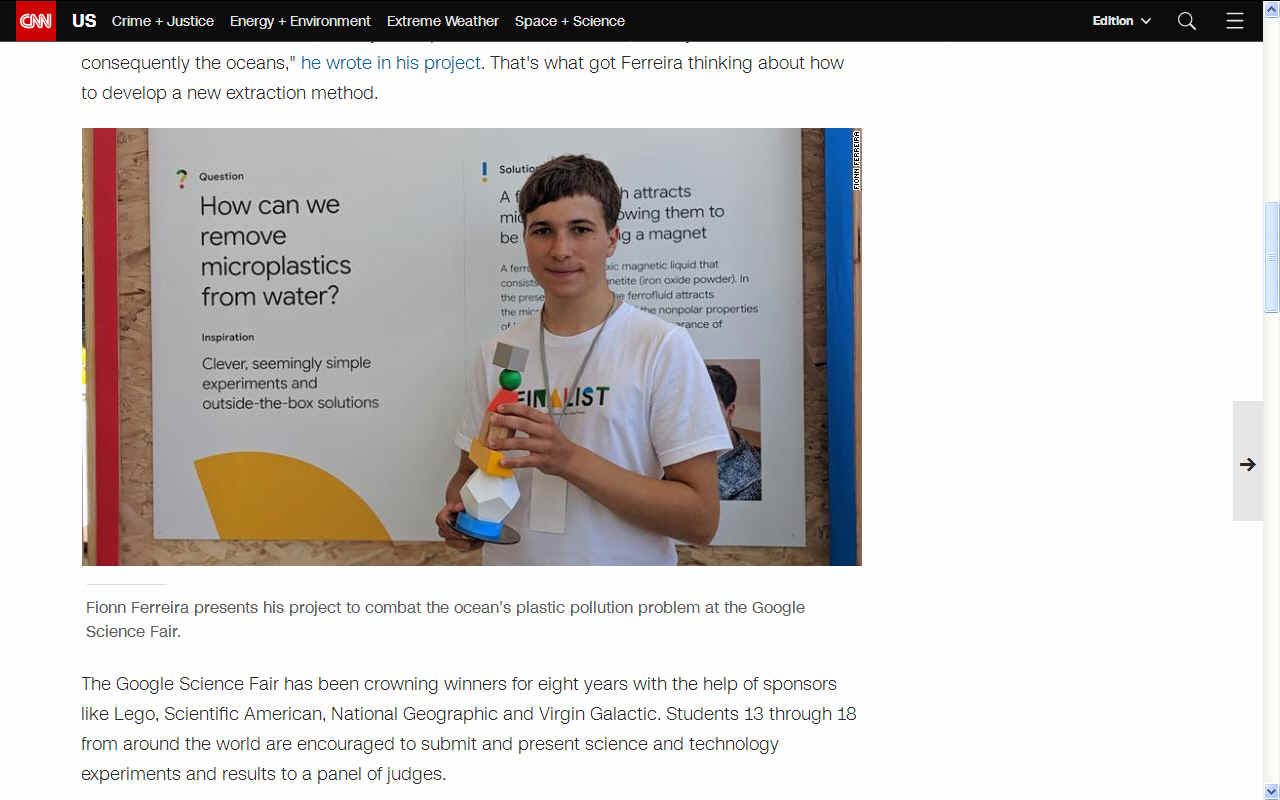
A walk on the beach led Fionn Ferreira to develop his project on microplastic extraction from water for the annual Google Science Fair. The project won the grand prize of
$50,000 in educational funding at this year's event.
MOTIVATED - According to his website, Fionn is motivated and enthusiastic, enjoys innovation and cool science, especially when things go bang! He has entered the BT Young scientist competition many times and loves inventing and tinkering at home. He has won awards with his science projects at both national and international science fairs and he worked at Schull Planetarium where he shares knowledge with others. Fionn can use the graphic design software Adobe Illustrator and Adobe Photoshop. He also codes in C++ and thinks it is really interesting to automate his home with Arduino and Raspberry Pi projects.
How to remove microplastics
When the microplastics latched on to the ferrofluids, Ferreira dipped a magnet into the solution three times to remove both substances, leaving clear water.
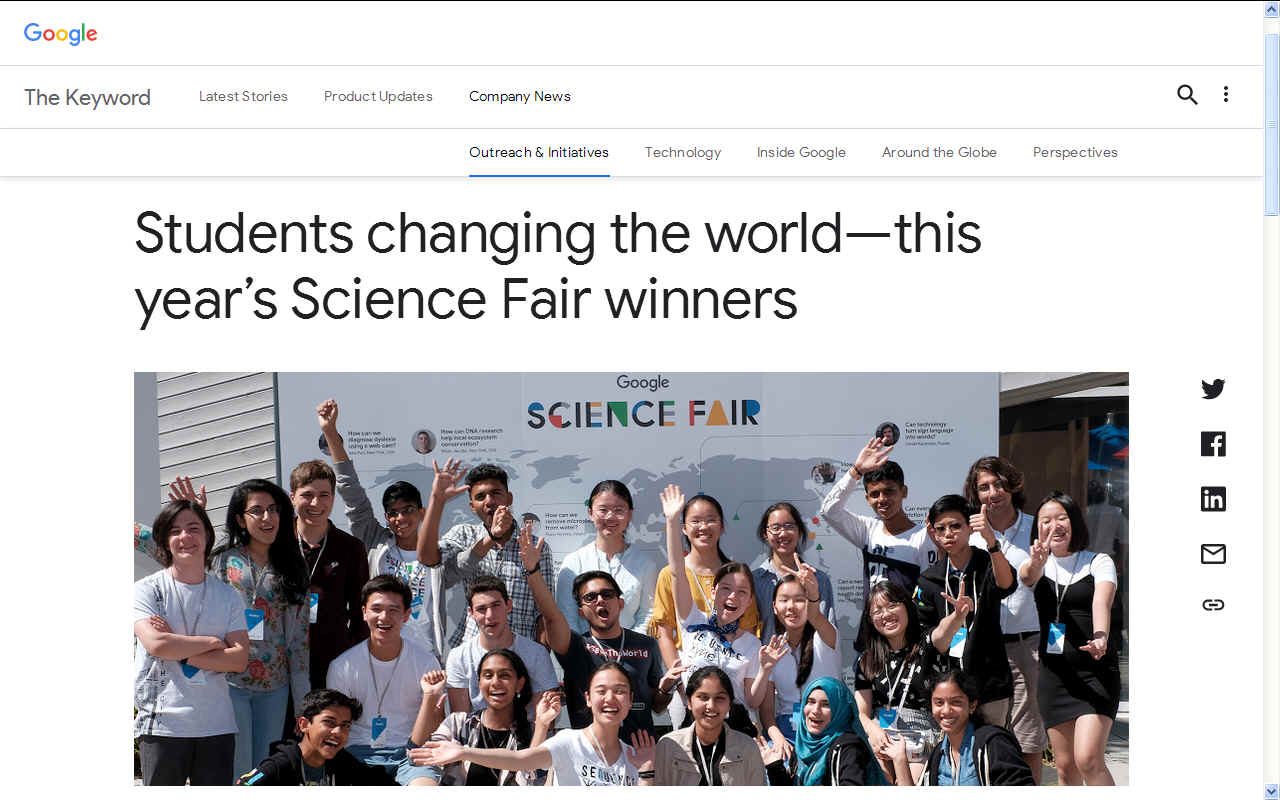
GOOGLED - Prize winners Fionn Ferreira and Celestine Wenardy at the science fair.
What's next?
Ferreira said his parents are happy at his accomplishment
- and because they don't have to fund as much of his college tuition, thanks to the prize money.
In 2019 the winners were:
Grand Prize: Fionn Ferreira - a West Cork, Ireland resident who wants to help save the oceans by extracting harmful microplastics from wastewater.
Virgin Galactic Pioneer Award: Celestine Wenardy - a student from Indonesia who set out to find affordable, non-invasive ways for members of her community to test their blood sugar levels
Scientific American Innovator Award: Tuan Dolmen - a Turkish science enthusiast who found a way to harness energy from tree vibrations.
National Geographic Explorer Award: Aman KA and AU Nachiketh - two young scientists from India who found an eco-friendly way to coagulate rubber.
Lego Education Builder Award: Daniel Kazanstev - a Russian student who wanted to find a better way to help those with impaired hearing communicate with the world around them.
FIONN'S PROJECT SUBMISSION
This project investigates a new method for the extraction of microplastics (plastic particles less than 5mm in diameter) from water. At present, no screening or filtering for microplastics takes place in any European wastewater treatment
centres 1.
IRELAND - The young Irish innovator has found a way of extracting microplastics from water using an emulsion of oil and magnetite. If the experiment can be scaled up for commercial extraction, perhaps using renewable energy, such as to make the process economically viable he may be onto a winner. Congratulations for your work so far Fionn.
Method / Testing and Redesign
Micro-plastic Production:
* Microplastics in cosmetic products were separated from the gels using suction filtration and desiccation. (LDPE).
* Plastic fibres were used from model making grass (nylon and polyester) and by removing plastic from the washing machine filter.
Preparation of microplastic suspensions:
The Microplastic extraction process:
The extraction process can be summarised as follows: -
0.5g of the magnetite powder was added to the test tube. -
The desired amount of oil (if any) was added to the tube. - The tube was stoppered and inverted 20 times to allow the magnetite and oil to cling on to the plastic.
- The stopper was removed and the ferro-fluid containing microplastics was removed using Neodymium magnets in a small test tube. Then the magnets were pulled out of the suspension, the ferro-fluid was removed from the tube by removing the magnets from inside the tube allowing the magnetite to fall off into a waste container.
-
The magnets were dipped into the suspension three times. - The sample was then ready for analysis.
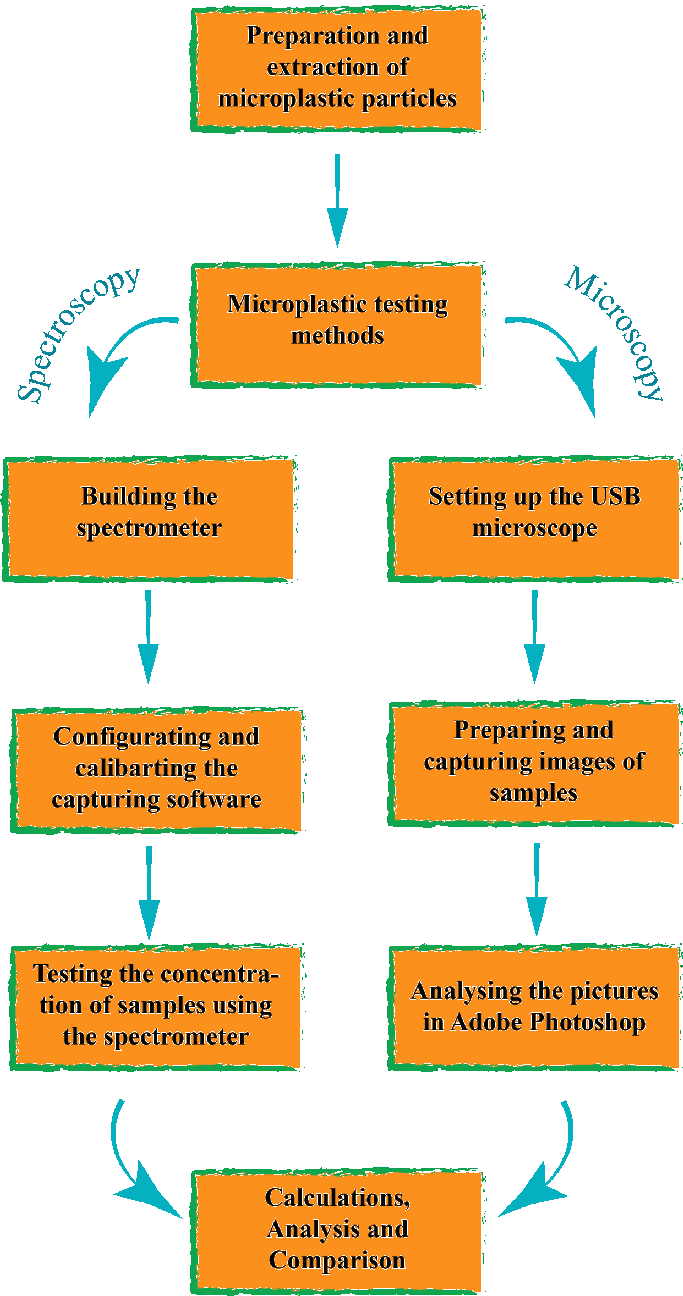
Testing the concentration of microplastics left behind after extraction
Two main methods were used to test the efficacy of the microplastic extraction process. The two methods used were spectroscopy and microscopy.
Spectroscopy
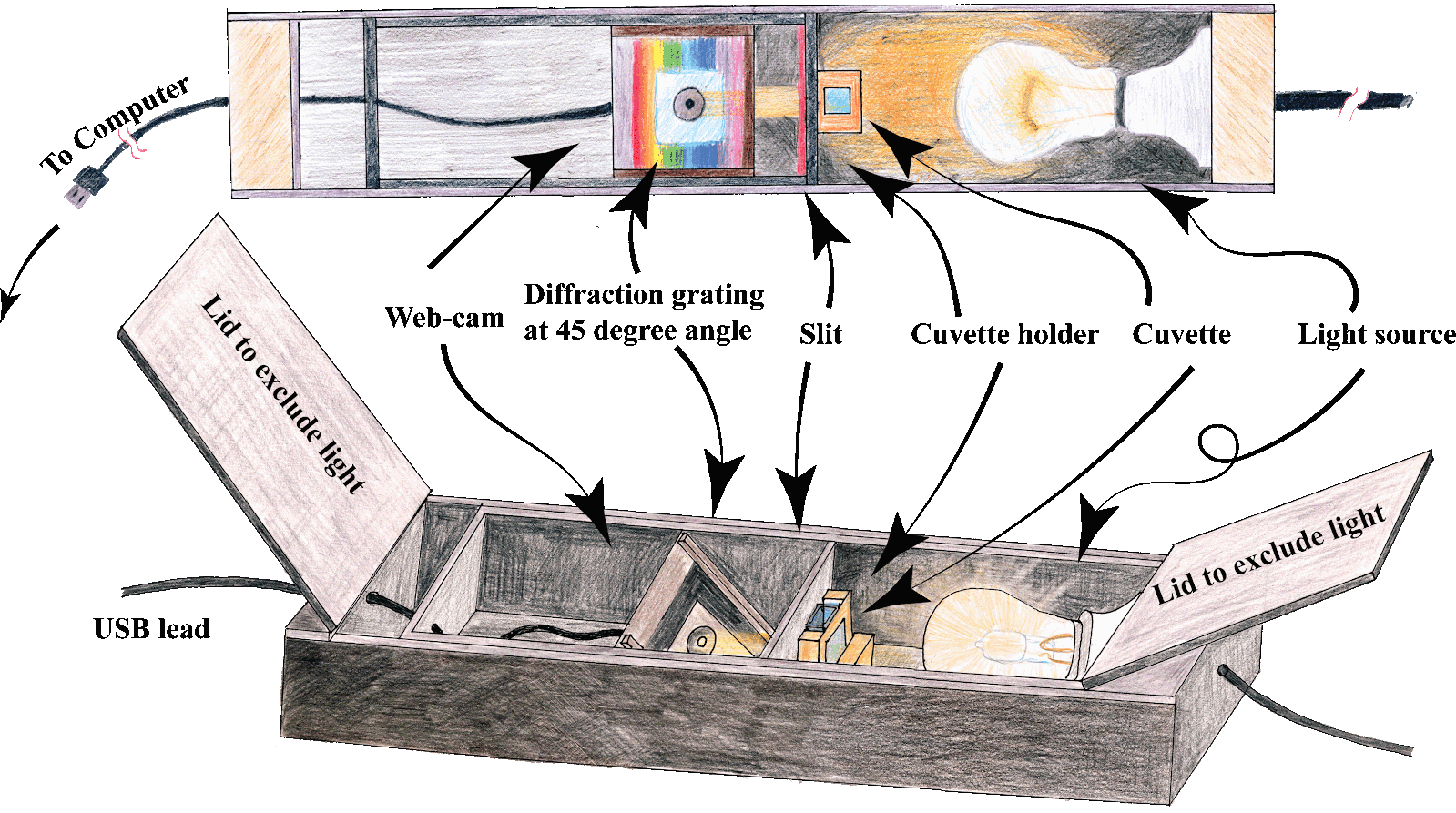
The first method chosen, was spectroscopy as I had built my own spectrometer. I live in one of the most remote areas of Ireland making it difficult to get tests done at professional labs. That is why I built my own.
After building many prototype spectrometers, I came up with a final spectrometer design which can be seen below.
I used the software spectragryph to analyse the spectra from the webcam. Spectra could be calibrated to wavelengths using a CFL lamp which has very distinct peaks of known wavelength.
Microscopy
As a secondary method, I used microscopy. I used a digital microscope with a phone screen as a light source. Like this, I could calibrate the image size using the phone pixels as a grid.
Safety
When preparing plastic samples, a dust mask and eye protection were worn due to the presence of plastic dust. In the extraction process, care was taken and eye and mouth protection were worn when handling the iron oxide powder to ensure that none was inhaled due to its very fine nature. Finally, in spectroscopy, the light source could get very hot and because of this, warning stickers were placed around the light box and it was always allowed to cool before use.
Results
10 different types of microplastic suspensions were tested. 3 extractions were carried out for each of the 4 different volumes of oil used in each extraction. To obtain a meaningful result, for each extraction, 50 spectrometer captures were carried out and 3 microscope captures were done. 120 captures in total were taken for every extraction. From these, means and standard deviations were calculated which allowed the further calculation of standard error and a final hypothesis test.
Results were obtained from both spectroscopy and microscopy. From spectroscopy, the % extraction of plastics could be calculated using the Beer-Lambert Law. here, 5 different suspensions of different concentrations were tested and the results were used to make a calibration graph. The absorption was calculated by obtaining the log (intensity(blank) / intensity (sample)) at a constant wavelength.
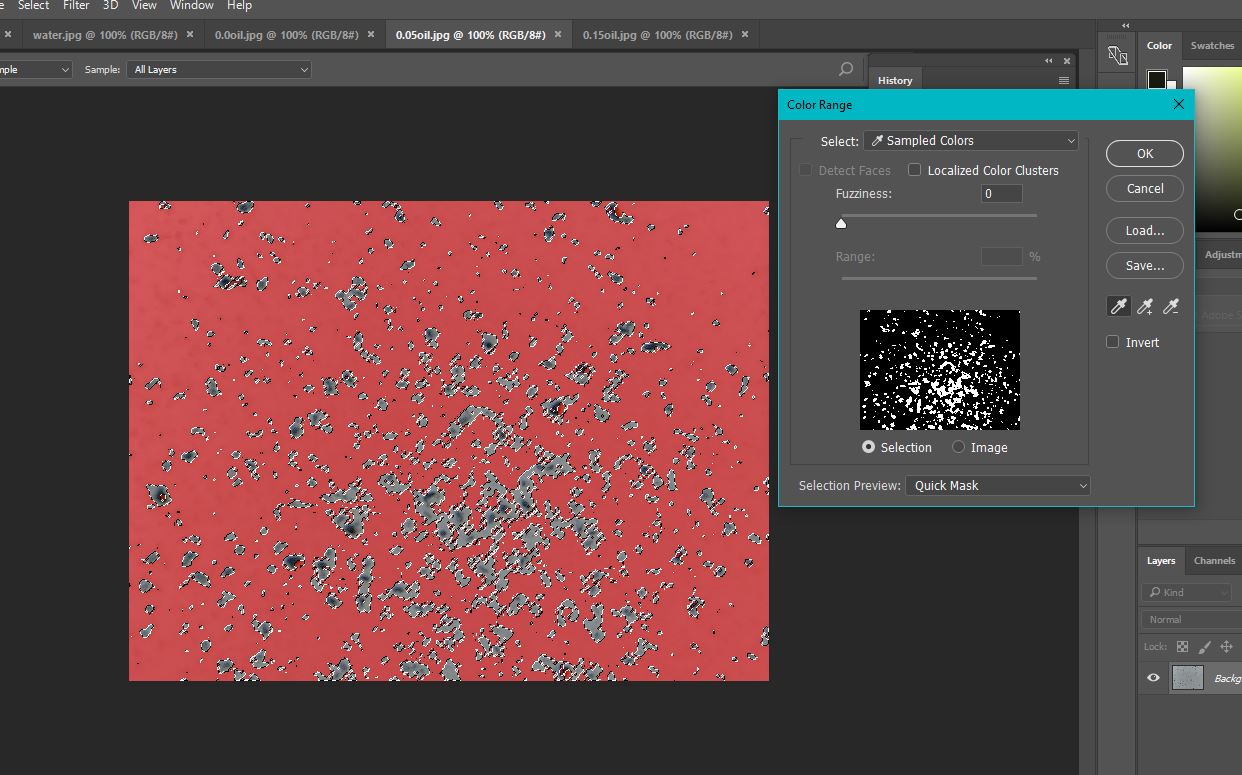
The results from microscopy were calculated by finding the % of each image covered by plastic (dividing the number of pixels covered by plastic by the total number of pixels and multiplying by a hundred). Then, from this, the % decrease was calculated.
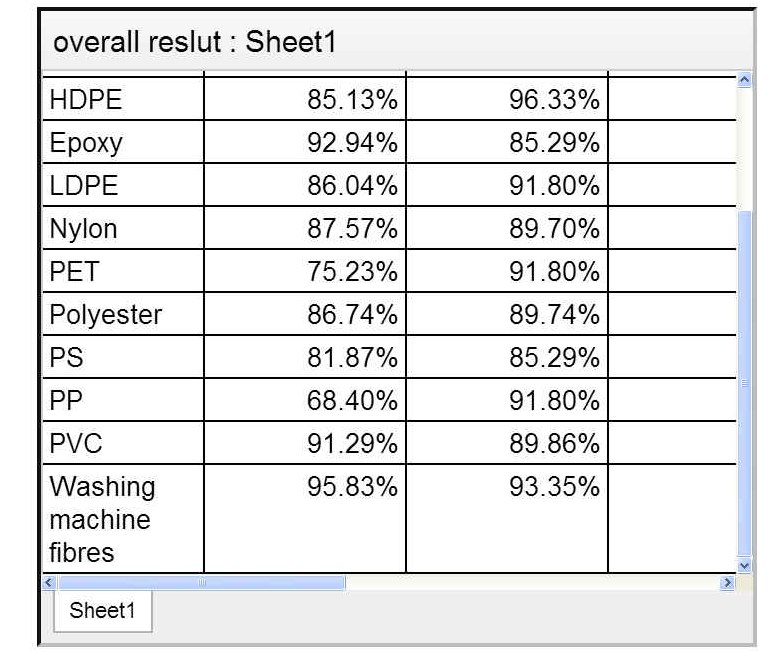
All types of plastics were individually analysed and graphed. This was all used to create a larger composite results table and graph.
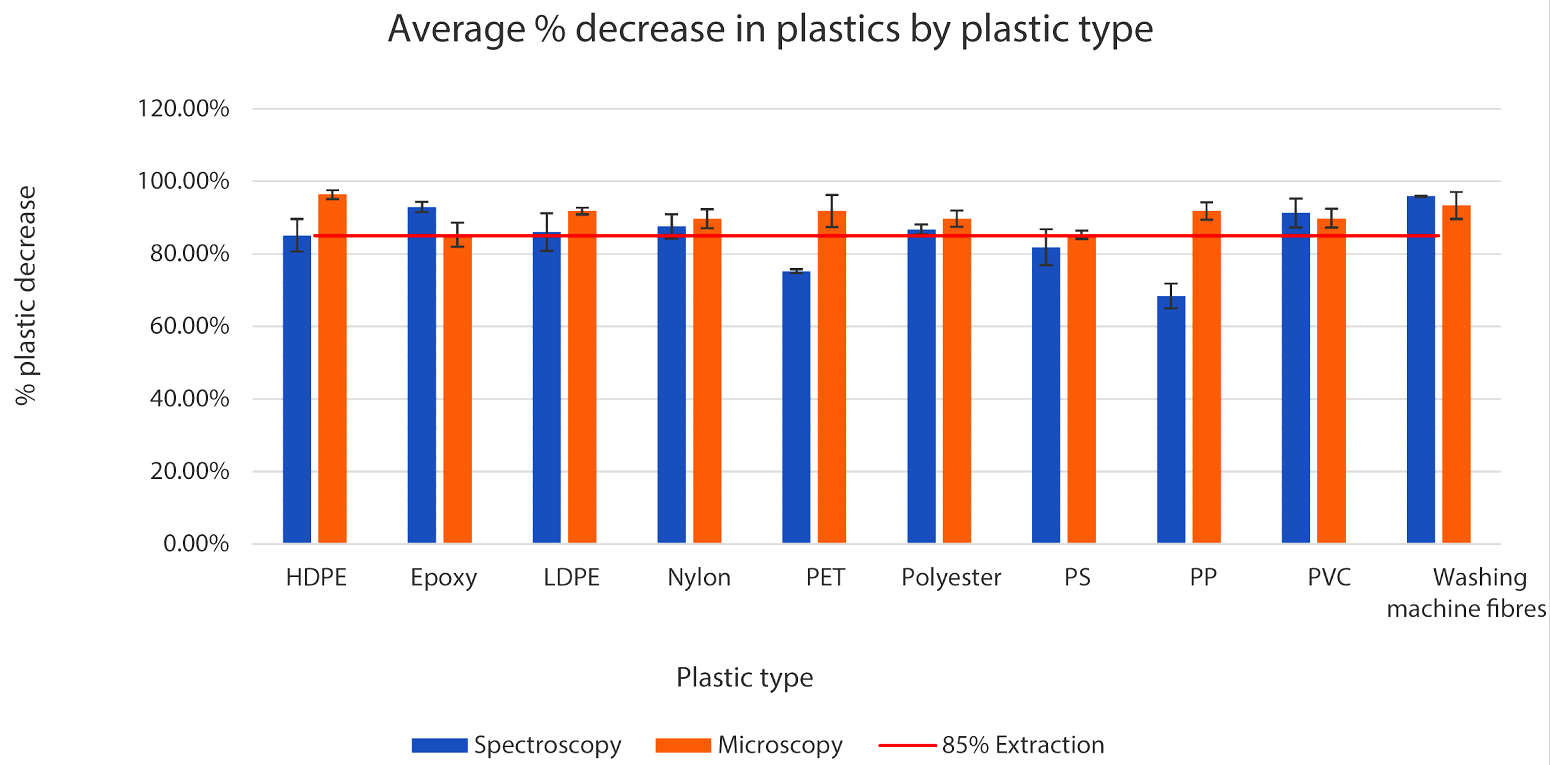
The graph below shows the average extraction rates for all of the plastics tested. An 85% extraction line is shown as this was the hypothesis I set out to investigate.
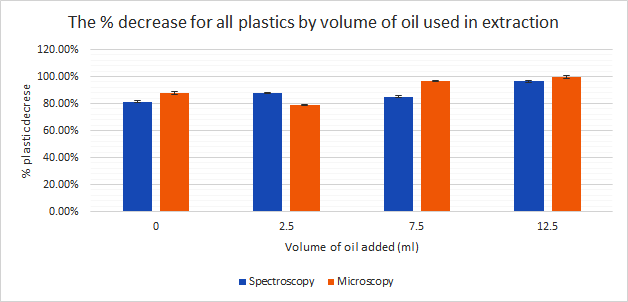
Another interesting comparison is the volume of oil used in extraction. I hypothesised that there would be an increased extraction rate with the increased volume of oil. A table of results showing the volume of oil used in extraction can be seen here.
The graph can be seen here:
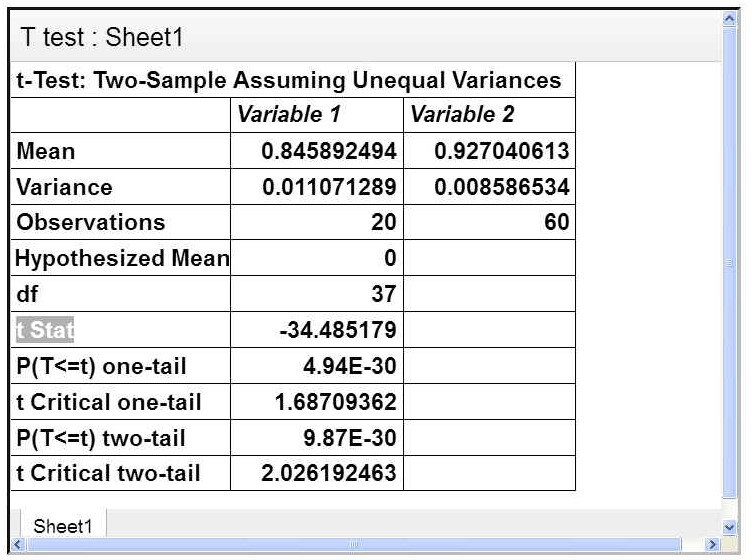
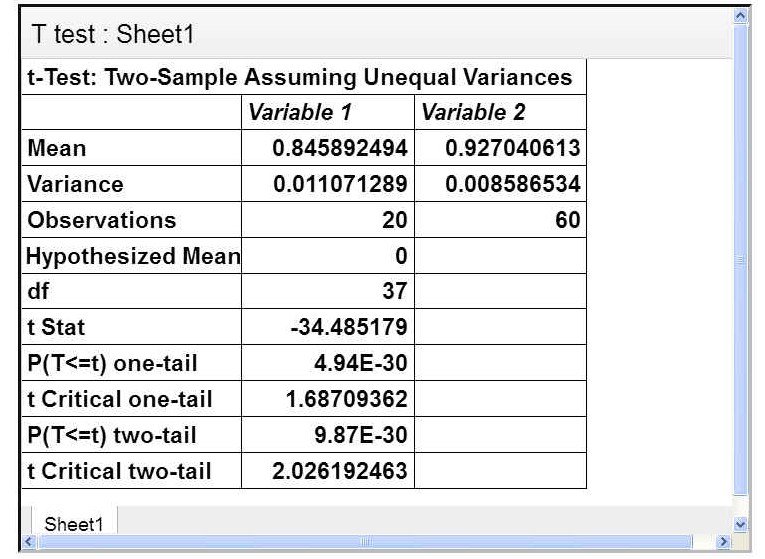
A statistical analysis was carried out to determine if there was a statistically significant difference between the efficiency of different volumes of oil used in extraction. The t-test, I carried out was a two-tailed test comparing the samples where no oil was used and the samples where oil was used. In the t-test, t stat was greater than t critical (2 tail), Hence I could conclude that there was no statistically significant difference between using oil or not using oil The test can be seen below:
Hypothesis test
From this test, I am 95% confident that the mean reduction of microplastic will lie between 86.6% and 88.7%
Conclusion
The following is a list of conclusions I have drawn from my experimental results:
For each plastic tested, the quantity of plastic removed was greater than 85% apart from Polypropylene which had an average reduction of 80% ± 3.07% (95% confidence).
The method was most effective for the plastic fibres extracted from the washing machine filter with an average reduction of 95% ±2.3% (at 95% Confidence) and HDPE plastic with an average reduction of 91% ±3.1% This shows that this method would be very useful in urban wastewater treatment plants, as over 55% of plastics in wastewater originate from washing machines and clothes 11.
On average, the tests with 12.5 ml oil/L water gave higher plastic reduction rates than tests without any oil added (magnetite only). However, the t-test carried out on these results showed no statistically significant difference. From this I can conclude that using magnetite with a minimum of oil forms a viable method for the extraction of microplastics. If this method is to be applied in wastewater treatment, I believe that it is important to minimise the amount of oil and magnetite used to make the extraction more economical while using enough to ensure the maintain high extraction rates to maximise plastic reduction.
There is no doubt that the most effective way to reduce microplastic pollution in oceans is to use less plastics and ensure that plastics used can be recycled and separated to prevent them from entering our wastewater, but the reality is that more and more of the products we use contain plastics and potentially degrade into microplastics before entering our wastewater. It is therefore essential that we find efficient and effective ways of extracting microplastics from wastewaters before they reach our watercourses and ultimately our oceans.
Once plastics enter our oceans, they are practically impossible to extract. The results of this project show that this could be a viable method. However questions remain to be answered. This project only forms the very beginning of this extraction idea which has never been conducted before. Further research needs to be carried out to investigate the efficacy of various grades of magnetite, different types of magnetic systems, methods for separating the waste and the design of a system that could be introduced into treatment centres.
About me
I really like science Youtube channels such as Nilered, The Backyard scientist, National Geographic and David Attenborough. I'm inspired by scientists such as Ben Ferringa and his work with nanochemistry and engineering. I'm in my last year of secondary school and would like to study chemistry or chemical engineering in Ireland or in Europe. I think that both of these subjects will be suited to me as I really enjoy problem-solving and experiments as well as engineering and tinkering.
One of the most valuable rewards from this science fair for me is the opportunity to present my work to a body of professional interested people. Winning any prize would be a great honour to me as this would be an acknowledgement of my project and ideas. This is especially true as I know that there are many bright young scientists competing with me. Also, winning a prize would give my project more attention and let it grow with mentorship to solve a real problem on the Earth. There is nothing I would like to see more than my project and idea to be used in real life applications and I think a prize could do this.
Health & Safety
All experimentation was conducted at home.
Plastic production:
When sanding plastics, a dust mask, safety glasses and a lab-coat were worn to prevent the possible inhalation of microplastic dust and the entering of the dust into the eyes.
The aspirator pump can cause the Buchner flask to implode. This can be a very dangerous experience. To prevent this from becoming a hazard, a metal cage was placed around the suction filtration apparatus and safety glasses were worn.
Extraction
Magnetite is a very fine powder and can lead to irritation to eyes and the lungs if the powder is inhaled. To prevent this, safety glasses and a dust mask were worn while handling the powder. Extracted ferro-fluid was stored in a glass container for further experimentation.
Spectroscopy
The light source is hot and can damage eyes if looked at directly, to avoid this, the bulb was allowed to cool before changing and the bulb was not looked at when turned on.
My mentor and supervisor who watched over me while I used any hazardous parts of the project was Anke eckardt: ankeckardt@gmail.com, 00353 (0)86 172 5550
Bibliography, references, and acknowledgements References:
https://www.youtube.com/watch?v=LV9209axVUs
https://ecologycenter.org/factsheets/adverse-health-effects-of-plastics/
Acknowledgments
Dr Fredrich Menges, Developer of the spectragryph software who gave me a free liscence to use the software
Anke Eckardt (Parent), who morally supported me throughout the 1000 tests and made lots of cups of hot chocolate!
Dr Ian O Connor, Researcher at GMIT Galway. He gave me valuable feedback after asking him for help with microscopy and general research.
Julie Mc Mahon (English teacher) teaching me how to write and express my science through a report.
Davoren Leung (Maths Teacher) who gave me extra time to show how to do a hypothesis test.
CONTACTS
Ballydehob, Co Cork, Ireland
OCEAN CLEANUP PROJECTS
* Adidas * Algalita research foundation * Aliance to end Plastic Waste * Boyan Slat's ocean booms * Fionn Ferreira's ferrofluid extraction of microplastics * LEGO Eagle Tech Titans, micro plastic robot, Texas * 4Ocean recycled plastic bracelets * Plastic Oceans Canada * Plastic Oceans Org * Seabin * SeaVax autonomous drones * Surrey University PIRATE & Triton
LINKS & REFERENCE
https://www.thejournal.ie/irish-student-science-award-microplastics-4745270-Jul2019/ https://www.googlesciencefair.com/ https://www.facebook.com/fionn.ferreira https://www.fionnferreira.com/ https://edition.cnn.com/2019/04/16/health/ocean-plastic-study-scn/index.html https://edition.cnn.com/2019/08/01/us/irish-teen-wins-google-science-fair-trnd/index.html
|
|||
|
ABS - BIOMAGNIFICATION - CANCER - CARRIER BAGS - COTTON BUDS - DDT - FISHING NETS HEAVY METALS - MARINE LITTER - MICROBEADS - MICRO PLASTICS - NYLON - OCEAN GYRES - OCEAN WASTE PACKAGING - PCBS - PET - PLASTIC - PLASTICS - POLYCARBONATE - POLYSTYRENE - POLYPROPYLENE - POLYTHENE - POPS PVC - SHOES - SINGLE USE - SOUP - STRAWS - WATER
PLEASE USE OUR A-Z INDEX TO NAVIGATE THIS SITE
|
|||
|
This website is provided on a free basis as a public information service. copyright © Cleaner Oceans Foundation Ltd (COFL) (Company No: 4674774) 2019. Solar Studios, BN271RF, United Kingdom. COFL is a company without share capital.
|